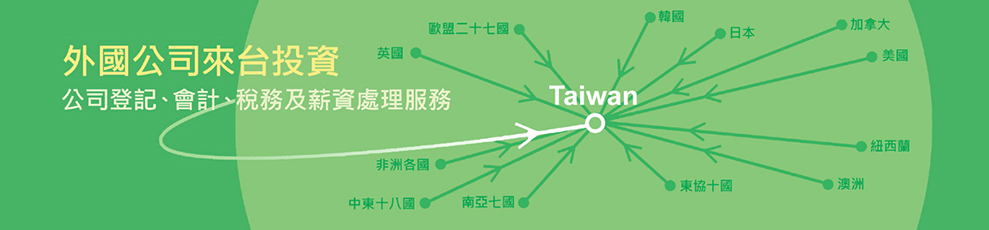
- 首頁
- 關於永輝
- 工商與特許
- 薪資與勞動
- 會計與資料處理
- 跨境稅務協定
- 台灣企業支付費用給海外企業的扣繳稅率20%相當高,在什麼情況下可以降低稅率或零稅率?
- 臺灣DTA零扣繳稅率豁免申請服務
- 台灣-所得稅法第25條第1項申請服務 tpe4ww.25
- 臺灣稅務優惠申請TSI第 15 - 1 條
- 台灣企業匯款給海外公司,要扣繳多少稅額?
- 台灣支付給海外公司智慧財產權授權金之扣繳問答
- 台灣稅務居民證相關問答
- 台灣與其它國家的租稅協定
- 台灣與中國的稅收協定
- 台灣與日本的稅收協定
- 台灣與英國的稅收協定
- 外籍航空公司在台灣分公司稅務問答
- 台灣移轉訂價 Taiwan Transfer Pricing
- 海外匯回台灣DTA零扣繳稅率豁免申請服務
- 外國公司在台灣以非居民(Non-Resident)身份運營稅務(VAT &CIT)法規問答
- Taiwan 境外電商課稅專區
- 2023年起台灣CFC受控外國公司稅務問答
- 台灣母公司透過香港轉投資持大陸孫公司,盈餘分配回台的抵稅規定
- 新加坡股東投資台灣公司獲利回收的稅負問題QA
- 海外航空公司委託台灣公司維護飛機的稅務問答
- ESG解決方案
- 專利年費代繳
- 合夥人制度
- 台灣企業專項
- 會議資訊
- 網站導覽